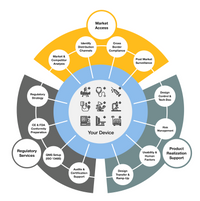
At adjutem, we empower innovators in the medical device and in vitro diagnostic (IVD) space to confidently navigate global regulatory landscapes. As a flexible and expert-driven consultancy, we support manufacturers throughout the entire product lifecycle—from development and documentation to successful market entry and post-market compliance.
- Finding contract manufacturing solutions
- Quality management system implementation
- Post-market surveillance and vigilance planning
- Regulatory intelligence and ongoing compliance support
Let us help you accelerate your product's journey to market. With a personalized approach and global regulatory insight, we are your partner in success—today and throughout your device’s lifecycle.
Together with our business partners we can offer a full coverage of product realization activities including development, production and regulatory services.
Reach out today to learn how we can support your next step.